WFI in bulk is ready from water or from purified water by distillation in an equipment of which the areas in connection with water are of neutral glass, quarts or acceptable metallic & which can be equipped with an efficient product to stop the entrainment of droplets.
Floor Qualities are regarded with developing interest due to the fact their functions meet up with the necessities in check out of more dependable in vitro assessments dependant on 3D aggregates, an progressive technique in comparison with standard types [27,28]. Spheroids, that are three-dimensional aggregates of cells, supply a a lot more physiologically appropriate design for learning mobile habits as compared to conventional two-dimensional cultures.
Sterile water for injection: It’s specs are offered in USP monograph for water for injection, sterilized and packaged in suitable single-dose containers, if possible of type I glass, of not much larger than one thousand ml dimension.
Creating tablet-sort medications: Purified water is used while in the preparing of capsule-sort medications that happen to be administered orally and absorbed within the human method.
Bacteriostatic WFI: This is certainly sterile Water for Injection that contains bacteriostatic (antimicrobial) agents. It may be packed in one-dose containers of not bigger than 5 ml dimension and in numerous-dose containers of not bigger than 30 ml sizing, the label of which indicates the identify as well as the proportion of extra agent.
The pH attribute was sooner or later acknowledged to generally be redundant into the conductivity check (which included pH as an aspect of the take a look at and specification); for that reason, pH was dropped for a independent attribute take a look at.
An archaic comprehension of microbial retentive filtration would lead a person to equate a filter's score with the false impression of an easy sieve or display screen that Certainly retains particles sized at or higher than the filter's ranking.
The development of RO models which can tolerate sanitizing water temperatures as well as operate effectively and consistently at elevated temperatures has added significantly for their microbial control also to the avoidance of biofouling.
. MICROBIAL ENUMERATION CONSIDERATIONS The target of the water procedure microbiological monitoring application is to supply adequate information to regulate and assess the microbiological good quality in the water made. Merchandise top quality specifications really should dictate water top quality requirements. An proper standard of control can be managed by using information website trending techniques and, if vital, limiting specific contraindicated microorganisms.
Methodologies that could be prompt as generally satisfactory for monitoring pharmaceutical water programs are as follows. Having said that, it should be mentioned that these are definitely not referee procedures nor are they essentially exceptional for recovering microorganisms from all water techniques.
Nonetheless, when coupled with regular thermal or chemical sanitization technologies or Found quickly upstream of a microbially retentive filter, it's handiest and may prolong the interval amongst process sanitizations.
Consequently, it might not be needed to detect the entire microorganisms species current in a specified sample. The monitoring plan and methodology need to point out adverse developments and detect microorganisms which are perhaps unsafe on the concluded product, course of action, or consumer. Closing collection of strategy variables need to read more be according to the individual prerequisites with the technique staying monitored.
Moistening air: Pharmaceutical cleanrooms demand sterile humidification. Incorrect humidity control can disrupt the manufacturing procedures and produce irreparable contamination.
The USP defines acceptable signifies of manufacturing the various types of ingredient waters. USP WFI may be made only by distillation or reverse osmosis.
 Alana "Honey Boo Boo" Thompson Then & Now!
Alana "Honey Boo Boo" Thompson Then & Now! Ariana Richards Then & Now!
Ariana Richards Then & Now!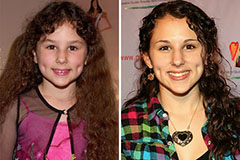 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Andrea Barber Then & Now!
Andrea Barber Then & Now! Burke Ramsey Then & Now!
Burke Ramsey Then & Now!